Why is dissolved oxygen important to aquatic life?
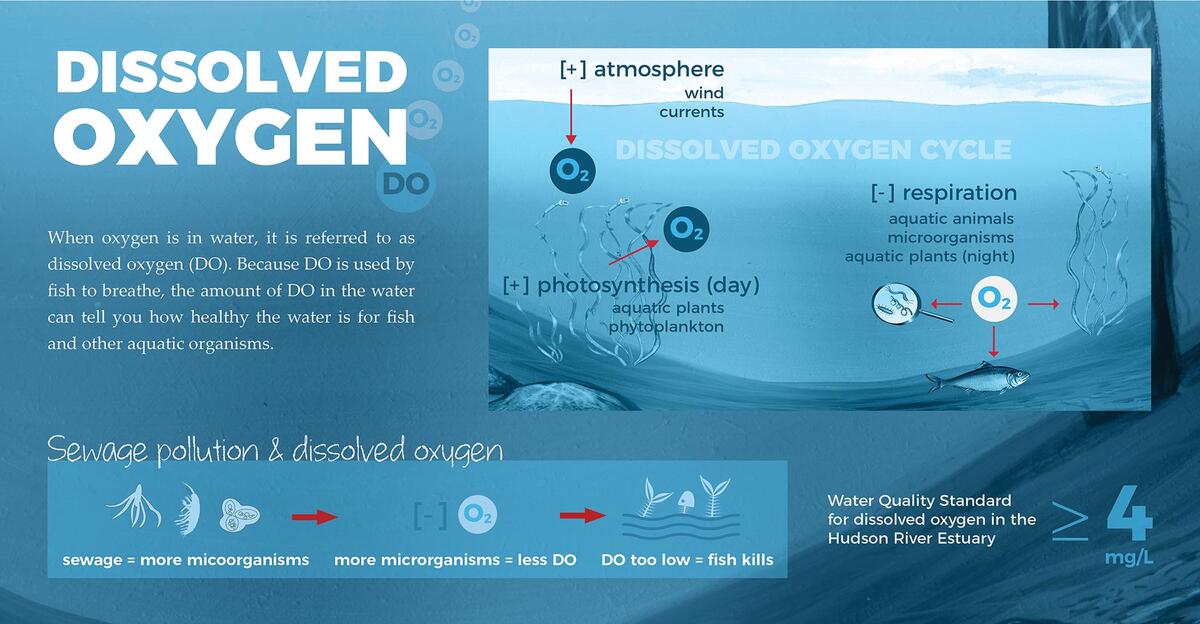
Like us, fish have to breathe oxygen, but they get it from the water. The oxygen they use is dissolved in the water, in much smaller quantities than what is in the air. Animals and plants do not use the oxygen from the molecular structure of water (H2O), but rather, they use the oxygen gas that is dissolved in the water.
Oxygen is important for many living things and for many of the chemical processes that happen in the water. There are two ways that dissolved oxygen enters water, either from photosynthesis from aquatic plants or through diffusion with the surrounding air.
Oxygen is also consumed in the water by respiration of aquatic animals and plants, decomposition of organic matter by microorganisms, and different chemical reactions. The combined oxygen consumed by all of the biological processes is called ‘Biochemical Oxygen Demand’, or BOD for short. When more oxygen is consumed than produced, dissolved oxygen levels in the water will decline.
When water has high, relatively stable levels of DO, it is usually considered a healthy ecosystem, capable of supporting lots of different kinds of aquatic organisms. Organisms have to adapt to changing levels of dissolved oxygen, and if these are extreme, it can cause them stress.

The Hudson River Environmental Conditions Observing System (HRECOS) provides real-time data on dissolved oxygen in the Hudson River via an online dashboard.
What causes low levels of dissolved oxygen?
Low DO (usually called hypoxic) levels usually indicate pollution or some type of human-caused change
- Addition of organic waste in the form of sewage and animal manure, organic fibers from textile and paper processing, and food wastes. These organic materials are decomposed by microorganisms that use up oxygen.
- Addition of nutrients from fertilizers and agricultural runoff as well as through sewage. This causes lots of plants and algae to grow and then decay. The bacteria that decompose the plants consume oxygen during the decay process
- Changing the flow of the water through dams and water withdrawal (for irrigation, snowmaking, water supply, or cooling systems of electric or nuclear power plants). The reservoirs created through a dam may increase the temperature and reduce the amount of dissolved oxygen.
- Raising the water temperature through the removal of vegetation from stream banks, which increases the water temperature and therefore decreases the dissolved oxygen levels. Another way that temperature can be affected is through the release of heated water that was used to cool an industrial plant.
Natural processes also affect the dissolved oxygen levels
- Warm water holds less dissolved oxygen than cold water.
- The lowest levels of DO usually occur in the morning, because photosynthesis stops at night while respiration continues.
- Water at higher altitudes holds less oxygen.
- Fast-moving water generally has more oxygen than still water, because the movement mixes the air into the water. However, if the water is very turbulent, it may hold too much oxygen, causing stress to the aquatic organisms.
- Water with lots of aquatic plants have higher levels of dissolved oxygen, since submerged plants produce oxygen through photosynthesis. Also, as mentioned above, too many plants will ultimately reduce the DO levels, because of either night-time oxygen use by plants or the decay process that consumes oxygen.
Dissolved oxygen and the Hudson River
In the Hudson River there are other factors that affect DO levels. Along the banks where the water moves more slowly, an invasive plant called water chestnut has dramatically reduced the DO in the water, often causing an anoxic situation. Anoxic means that the water doesn’t contain any dissolved oxygen.

Some animals, like carp and catfish, can survive with less oxygen, while others, such as salmon and trout, require more. When DO becomes scarce, some animals may be able to obtain their oxygen directly from the surface, such as the diving beetle, frogs, or even catfish. Testing water samples for DO will let you know if your water is healthy and can sustain life.
Measuring dissolved oxygen: How much is needed to support life?
Dissolved oxygen is measured in a few different ways: parts per million (ppm), milligrams per liter (mg/L), or percent saturation. When measuring DO, concentrations range from 0 to 14 ppm or mg/L (they measure the same thing, but sometimes your test kit will use only one of the measurements), and 0-125+ percent saturation. When measuring percent saturation, you also need to know the temperature of the water, because that can change the result.
For mg/L:
0-2 mg/L: not enough oxygen to support most animals
2-4 mg/L: only a few kinds of fish and insects can survive
4-7 mg/L: good for most kinds of pond animals
7-11 mg/L: very good for most stream fish
For percent saturation:
Below 60%: poor quality, bacteria may be using up the DO
60-79%: acceptable for most stream animals
80-125%: excellent for most stream animals
125% or more: too high
Biochemical Oxygen Demand (BOD)
Biochemical oxygen demand, or BOD, measures the amount of oxygen consumed by microorganisms when they decompose organic matter in stream water. BOD also measures how much oxygen is used up by chemical reactions in the water. A test is used to measure the amount of oxygen consumed by these organisms during a specified period of time (usually 5 days at 20 C). The rate of oxygen consumption in a stream is affected by many of the same variables as were described above: temperature, pH, the presence of certain kinds of microorganisms, and the type of organic and inorganic material in the water.
BOD directly affects the amount of dissolved oxygen in rivers and streams. The greater the BOD, the more rapidly oxygen is depleted in the stream, because microorganisms are using up the DO. This means less oxygen is available to higher forms of aquatic life.
The consequences of high BOD are the same as those for low dissolved oxygen: aquatic organisms become stressed, suffocate, and die.
Sources of BOD include leaves and woody debris; dead plants and animals; animal manure; effluents from pulp and paper mills, wastewater treatment plants, feedlots, and food-processing plants; failing septic systems; and urban stormwater runoff. Adding oxygen to the stream water through rapids and waterfalls, for example, will accelerate the decomposition of organic and inorganic material. Therefore, BOD levels at a sampling site with slower, deeper waters might be lower for a given volume of organic and inorganic material than the levels for a similar site in highly aerated waters.
Chlorine can also affect BOD measurement by inhibiting or killing the microorganisms that decompose the organic and inorganic matter in a sample.




